Animal cloning technology using somatic cell nuclear transfer: Research orientation and application in livestock production in Vietnam
03/09/2025TN&MTAnimal cloning is the process of producing genetically identical individuals without undergoing conventional fertilization. The successful creation of cloned animals has opened up numerous potential applications in basic research, medicine, and agriculture.
Currently, two major methods of animal cloning are employed worldwide: cloning using embryos derived from embryonic cells, and cloning using somatic cell nuclear transfer (SCNT). Compared with cloning based on embryonic cells, SCNT offers several advantages. It can be applied to animals with known phenotypes; donor somatic cells are abundant and easy to obtain, thereby increasing the number of embryos and cloned animals that can be generated.
SCNT is the process of transferring the nucleus of a donor somatic cell into an enucleated oocyte (Figure 1). The birth of Dolly the sheep marked the first successful case of animal cloning using SCNT. Following this milestone, numerous reports have documented successful cloning of livestock species such as cattle, goats, and pigs.
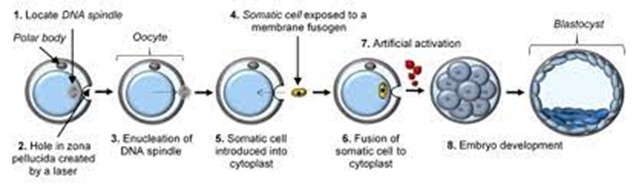
Figure 1. Somatic cell nuclear transfer process for producing cloned embryos
The development of SCNT technology has created new directions for both basic and applied research, such as producing transgenic animals, conserving and sustaining rare and endangered species, and generating animals with organs compatible for human xenotransplantation. SCNT is also being applied to breed animals with superior traits and to conserve species at risk of extinction.
Animal cloning by SCNT in Vietnam
The first studies on SCNT in Vietnam were initiated in 1998 at the Institute of Biotechnology. The Reproductive Biotechnology Group, led by Dr. Bui Xuan Nguyen, conducted SCNT research on several species, including cattle (2000), monkeys (2005), the saola (2005), and pigs (2006). However, the results were limited to the generation of cloned embryos.
In 2015, the National Key Laboratory for Animal Cell Technology at the Institute of Animal Science began research on embryo production and animal cloning using SCNT. In 2018, for the first time in Vietnam, the laboratory reported the successful production of zona-free cloned bovine embryos. Currently, zona-free SCNT is increasingly replacing traditional SCNT (zona-intact embryos) due to advantages such as simplicity, ease of manipulation, higher accuracy and efficiency, and the elimination of the need for expensive equipment. In March 2021, the laboratory successfully produced cloned male Vietnamese I pigs (I pig, a Vietnamese indigenous breed) using SCNT (Figure 2).

Figure 2. Cloned male Vietnamese I pig
These pigs were the first cloned animals in Vietnam to be produced from zona-free SCNT embryos. This achievemen represents a major scientific and technological breakthrough in animal cloning research in Vietnam. Since then, the laboratory has successfully generated cloned female Vietnamese I pigs (Figure 3). Both male and female cloned Vietnamese I pigs have demonstrated normal growth and reproductive capacity (Figure 4).

Figure 3. Cloned female Vietnamses I pig

Figure 4. Offspring born from cloned female Vietnamese I pig following artificial insemination with cryopreserved-thawed semen from cloned male Vietnamese I pig
Research orientation and applications of animal cloning technology in Vietnam
Application of animal cloning technology in the conservation and development of valuable livestock breeds
Maintaining, exploiting, and developing indigenous and valuable livestock breeds in an effective and sustainable manner, serving both economic development and genetic resource conservation while ensuring biodiversity, has become a global trend, including in Vietnam. Over past decades, many indigenous livestock breeds in Vietnam have deteriorated, become rare, or even disappeared, such as the fatty I pig, Thuoc Nhieu pig, and Son Vi chicken…
Scientific studies have shown that, if properly selected, bred, and raised according to technical protocols, indigenous breeds can achieve reasonable productivity and growth performance, making them suitable for development into specialty products characteristic of ecological regions across the country. Such products could meet domestic consumption demands in the context of deeper global economic integration.
Somatic cells serve as a valuable genetic resource widely used for conserving livestock breeds worldwide. Elite animals can be preserved, and their desirable traits, such as high milk yield, rapid growth, or disease resistance, can be rapidly disseminated. Applying SCNT for the conservation of rare livestock breeds also allows preservation of valuable genetic resources that are declining due to climate change, disease, or uncontrolled crossbreeding. Furthermore, SCNT enables the preservation and regeneration of valuable animals even after their death, an advantage unique to ex situ conservation (in vitro preservation of animal genetic resources) that in situ conservation (maintenance of live animal populations) cannot provide.
On this basis, it is essential to establish a strategy for building and developing a cryopreserved somatic cell gene bank to conserve genetic material from indigenous and valuable livestock breeds, particularly those at risk of extinction in Vietnam. Such a cryobank would provide a vital source of material for regenerating rare or high-value animals through cloning when needed.
Application of animal cloning in combination with gene editing for livestock breeding
In recent years, gene editing has emerged as a transformative technology for improving livestock genetics by enabling targeted deletion, addition, or modification of alleles within the genome. When introduced into zygotes or germ cells, these modifications can be stably inherited. Gene-edited livestock with improved genetic traits have been shown to be both scientifically and economically valuable.
Gene editing can be integrated into breeding programs to modify genetic variation and shorten generation intervals, thereby enhancing genetic gain. Globally, research on gene editing in livestock has focused on creating animals with enhanced disease resistance or production traits, such as increased muscle growth in cattle, goats, and sheep; improved wool length and density in sheep; modified milk composition; and enhanced reproductive performance.
CRISPR/Cas9-mediated gene editing has been performed in fibroblasts (somatic cells) in several laboratories worldwide. Gene-edited fibroblasts can be cultured into cell lines, which then serve as nuclear donors for SCNT. Gene-edited cloned animals thus combine valuable reproductive, growth, or disease-resistance traits with predictable genotypes. Compared with direct editing of embryos, this cloning–gene editing approach allows greater control over genetic outcomes. Consequently, integrating SCNT with gene editing to produce livestock tailored for specific purposes represents a strategic direction for the sustainable development of Vietnam’s livestock sector.
Application of animal cloning to produce laboratory animals for biomedical and pharmaceutical research
The essence of animal cloning lies in producing two or more individuals with identical genotypes and phenotypes. Such animals constitute ideal models for basic research, enabling precise studies of the effects of genotype or environmental factors on productivity or phenotype. They also serve as valuable resources for drug testing, pathology, and biotechnology research. Importantly, the cost of using genetically identical animal models is much lower than conducting research on genetically heterogeneous populations—for instance, two cloned calves can serve as a control group equivalent to, or even exceeding, that of 10–25 conventional calves.
Gene editing technology also makes it possible to develop cell lines capable of producing therapeutic proteins, antibodies, or enzymes. When these modified cell lines are used as nuclear donors for SCNT, the resulting gene-edited cloned animals can express valuable biomedical products. For example, gene-edited cloned cattle producing milk containing insulin, or cloned goats producing proteins for hemophilia treatment, have been developed. The creation of such cloned animals carries significant implications for biomedical and pharmaceutical research.
Assoc. Prof. Pham Doan Lan, PhD
Deputy Director, Institute of Animal Science